Dentistry employs many advanced materials and methods. Many adhesives and fillings are on the forefront of polymer science since they are expected to handle many years of physical stress in the rather chemically harsh environment that is in our mouths.
On top of this, they have to be non-toxic and be easily applied in a pain-free and convenient manner, where they are expected to solidify and set at exactly the right time.
Photochemistry is the method of choice for this, generally making use of short-wavelength visible or UV-A light to ensure that the curing process can be triggered exactly when wanted. This way, the chemical reactions that create our dental fillings can be completed in seconds.
Monitoring advanced reactions
Observing chemical reactions in real time is difficult. Mid-infrared spectroscopy offers the possibility to non-destructively monitor bond-specific changes at high speeds.
Currently, rapid-scan FTIR is used for similar investigations. This technique has three main limitations for such experiments: brightness, time resolution and spectral resolution. Learn more here about how the technology of IRsweep’s IRis-F1 can give better signal to noise ratios for faster measurements while retaining a very high effective spectral resolution.
IRsweep’s IRis-F1 spectrometer can measure a spectrum with a60 cm-1 spectral width in 1 μs. Here we measured the curing of dental resins and binders at a repetition rate of 100 spectra per second and a spectral resolution of 4 cm-1.
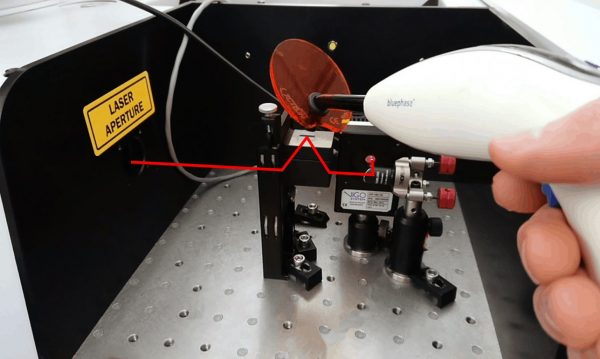
An IRUBIS silicon attenuated total reflection (ATR) crystal was used as a sensing substrate in the IRis-F1 spectrometer. This means the material directly on the surface of the crystal is measured. The reactions were initiated using a handheld fiber-optic coupled bluephase® curing light (Ivoclar Vivadent), which irradiated the substrate with light at wavelengths between 380 and 500 nm.
Results
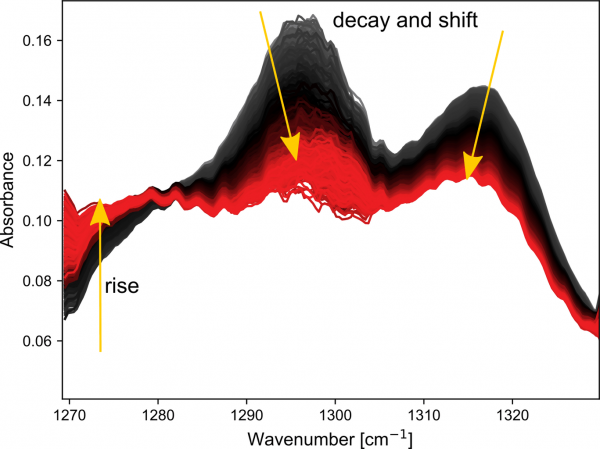
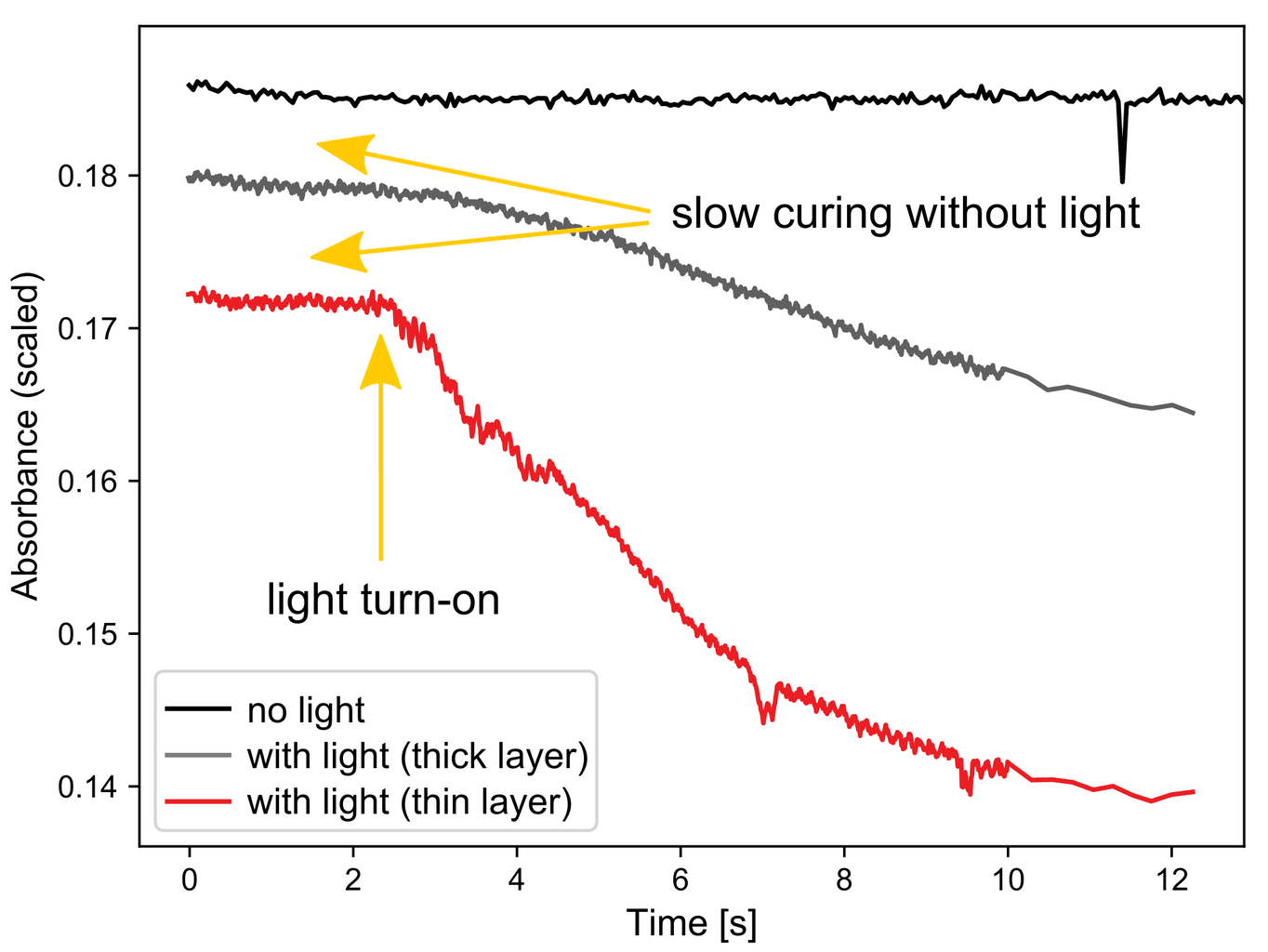
The first experiment investigated the Duo-Link two-component luting cement (BISCO). Clear spectral changes are seen during curing, manifesting in a decay and shift of the bands at 1295 and 1316 cm-1 and a rise at lower wavenumbers. Plotting these changes as a function of time shows the true power of using fast infrared spectroscopy for the analysis.
Two different thicknesses were investigated and it is clear from the time traces that the thin layer (red line) cures much faster than the thick layer (grey line). Ten seconds into the experiment the thick layer is still relatively uncured and light treatment would have to be continued to get a good result. The rate of curing strongly depends on the thickness of the layer.
The adhesive also shows some curing without light (black line), which is expected due to the “dual cure” feature of this product. Once the light is switched on, the spectral changes are rapid.
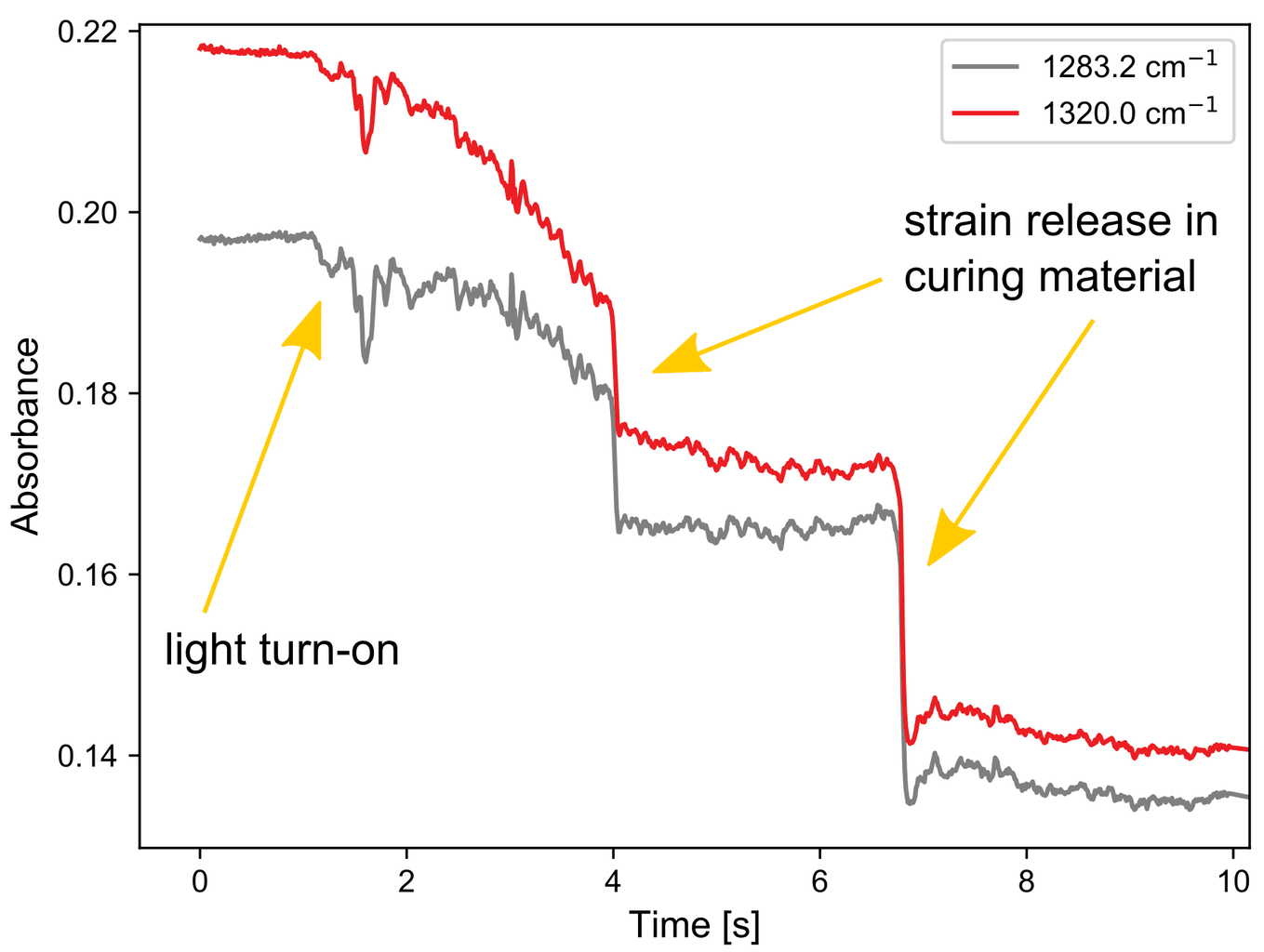
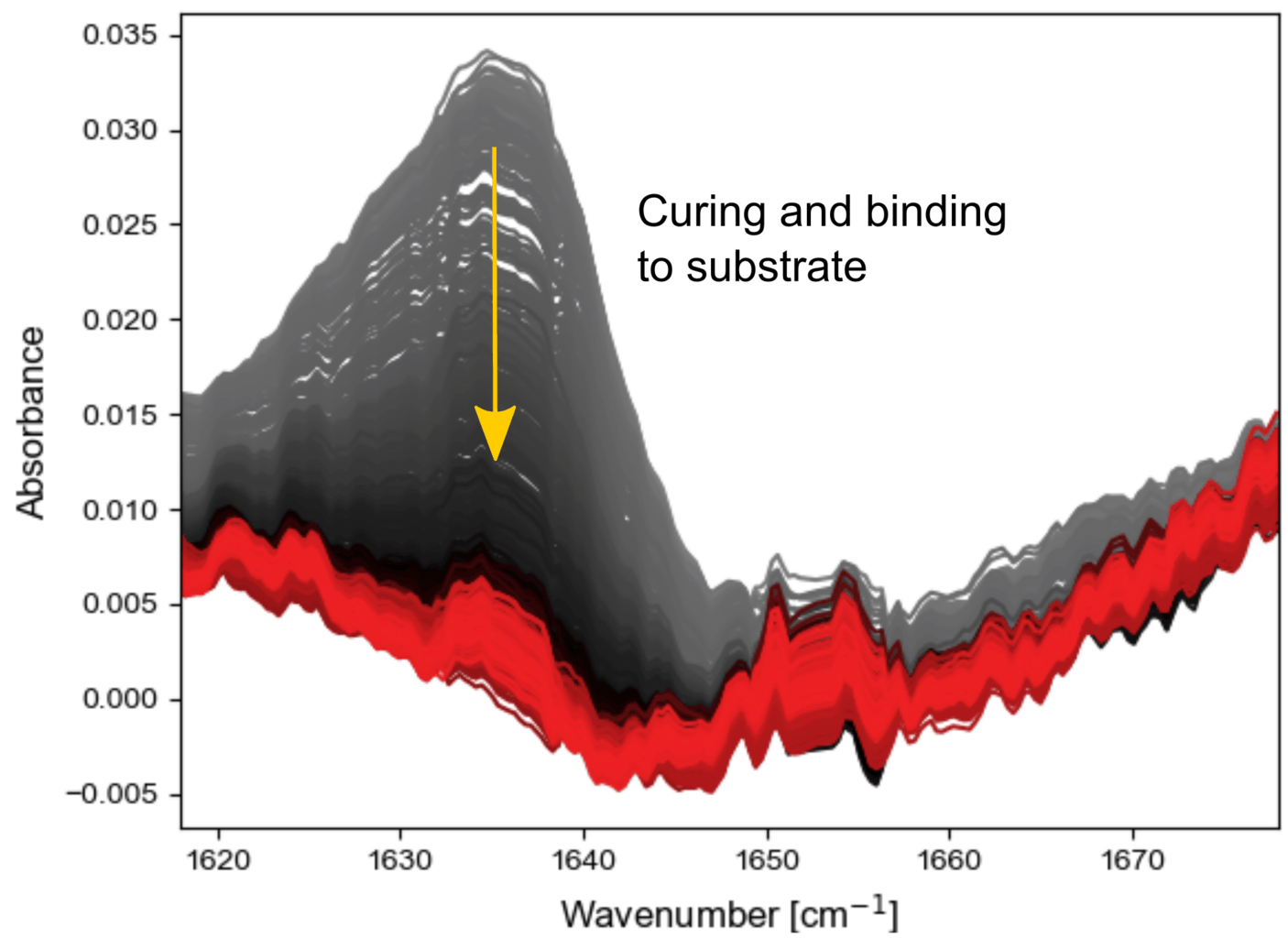
Not just chemical changes can be seen using IR spectroscopy but also physical changes, as shown in the following experiment. Normally this cement is used with an adhesive. Here it is intentionally left out, and no or incomplete binding to the substrate occurs.
This means that changes in the material, for example contraction/hardening, can lead to large changes on the measurement interface. As shown on the right, these events are easily followed with fast mid-IR spectroscopy.
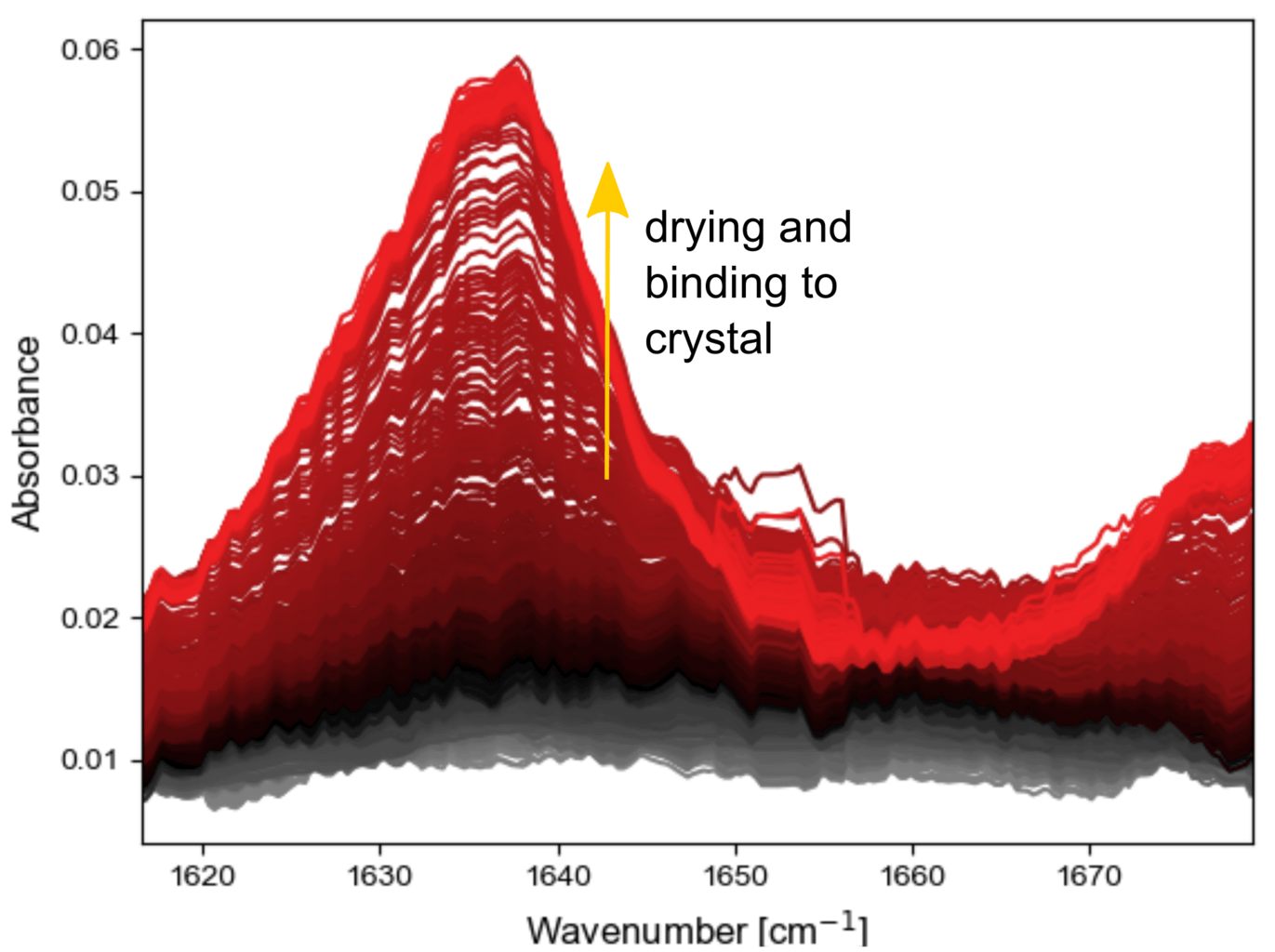
A final example is the drying and binding of the single component universal primer Monobond Plus (Ivoclar Vivadent). The product is designed to adhere a variety of materials, including silicates.
When it is investigated by itself on the silicon ATR crystal, a peak appears, corresponding to binding to the crystal (below, left). Interestingly, the heat from the lamp seems to strongly accelerate the reaction, since this is not a photo-activated material.
If a layer of Duo-Link is added on top of Monobond Plus before the light is turned on (below right), the curing reaction again proceeds as intended.
Summary
With the IRis-F1 it is possible to monitor fast reactions, ranging from timescales of milliseconds to minutes.
Curing of dental materials is an exciting field that can benefit from faster and brighter measurements. Chemical and physical changes have an effect on the resulting infrared spectra, meaning advanced analysis can be done even if no chemical changes are involved. We have found that the effects of the lamp will be very dependent on how exactly a dentist applies the layer of cement. On top of that, the light cause potentially unwanted effects on materials that are not designed to cure.
The technique is also non-destructive and can be adapted to many methods of sampling. This means it is possible to test these materials in realistic environments that are similar to where they will be used.
Keep reading
Want to know more?
Find out about industrial curing reactions, applications of the IRis-F1 to polymer chemistry, and the operating principles of dual comb spectroscopy.