Understanding the mechanism of a chemical reaction is a first step away from trial-and-error-based optimization.
By studying the structures of intermediates, for how long they exist, and the ways in which they can and cannot react, reactions can be intelligently designed and controlled.
The stopped flow technique
Stopped flow is a powerful technique to study reactions on a millisecond to minute timescale. Simply speaking, it works by rapidly mixing reagents before a spectroscopic cell, as depicted in the figure.
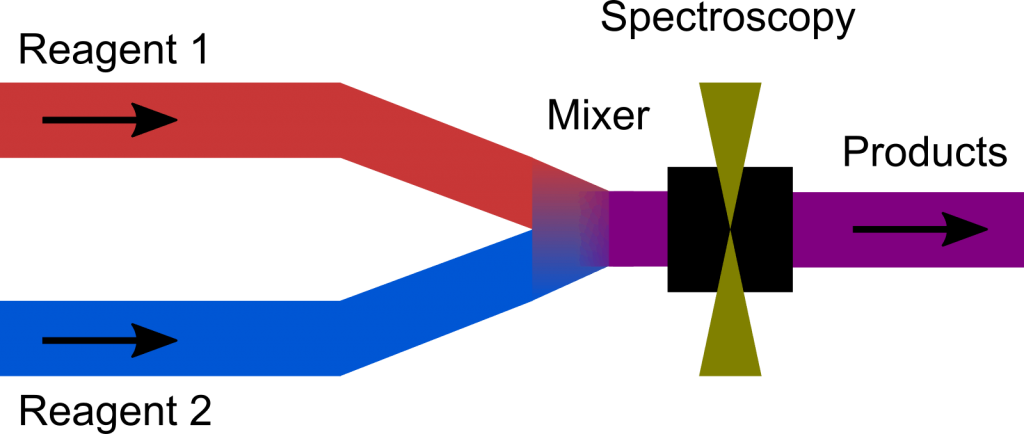
After mixing, the flow is stopped and the resulting chemical reaction after the mixer can be analysed using a variety of techniques. The time resolution is either limited by the mixing or the spectroscopy, whichever is slower.
UV-visible or fluorescence spectroscopy have the advantage that fast reactions can be studied, either at single wavelengths or with an array detector. This allows the determination of accurate kinetic information. On the other hand, it is difficult to determine structural information from UV-visible techniques.
IR spectroscopy is much more structurally sensitive, but fast kinetic determination at a single wavelength is not possible with modern Fourier-transform instruments; due to the nature of the technique, the entire spectrum is necessarily acquired every time. Rapid scan instruments start to address this by measuring entire spectra at high speeds, but this technique suffers from low signal-to-noise ratios and is still not fast enough for state of the art commercial stopped flow equipment.
Experimental setup

A picture of the experimental setup is shown. The spectroscopic interface was an attenuated total reflection (ATR) device in the IRis-F1 sample compartment. A mixing tee was placed directly before the ATR and the solutions were pumped by hand using syringes.
We previously used the laser based IRis-F1 mid-IR spectrometer to monitor sub-second curing reactions at 40 spectra per second. In the meantime we have upgraded the software of our spectrometer to operate at 150 spectra per second.
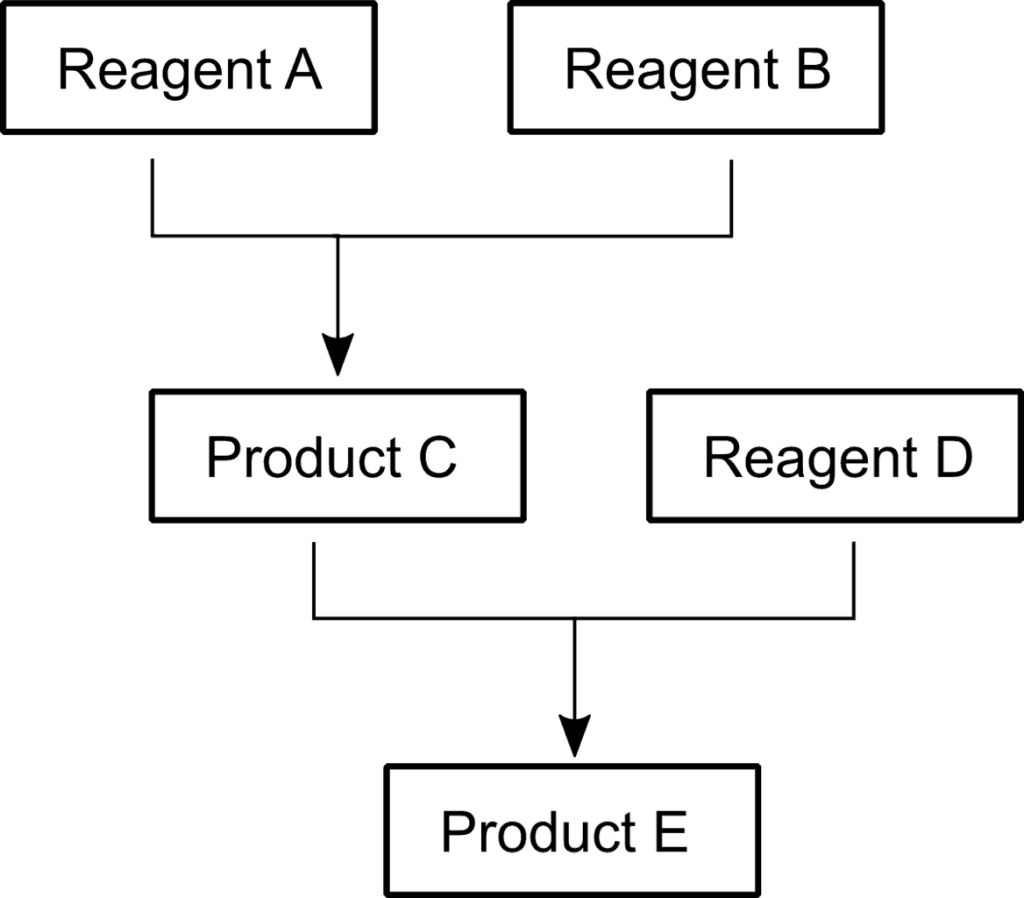
This experiment explores the possibility of monitoring a multi-step reaction in a stopped flow setup. A future experiment is also planned with more sophisticated flow controllers, where high speed is much more important.
The reactions investigated are depicted in the scheme. Reagent A, Product D and Product E all have bands in the 1600 to 1700 cm-1 range and were measured with the same laser.
Results
The results of the first reaction investigated, Reagent A + Reagent B → Product C, are shown in the figure.
All spectra shown are with a spectral resolution of 2.7 cm-1.
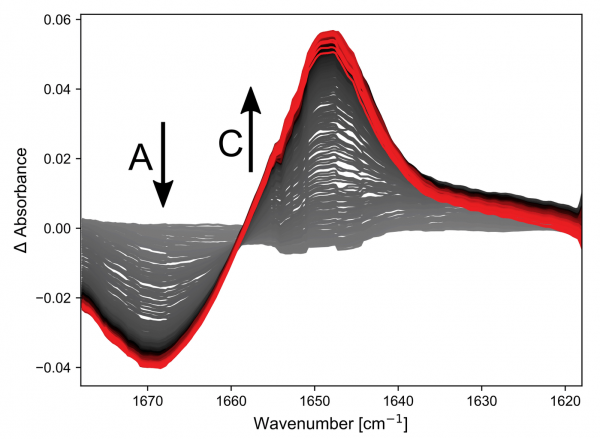
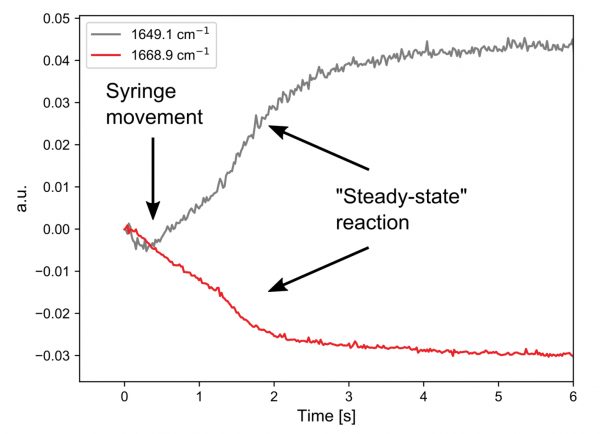
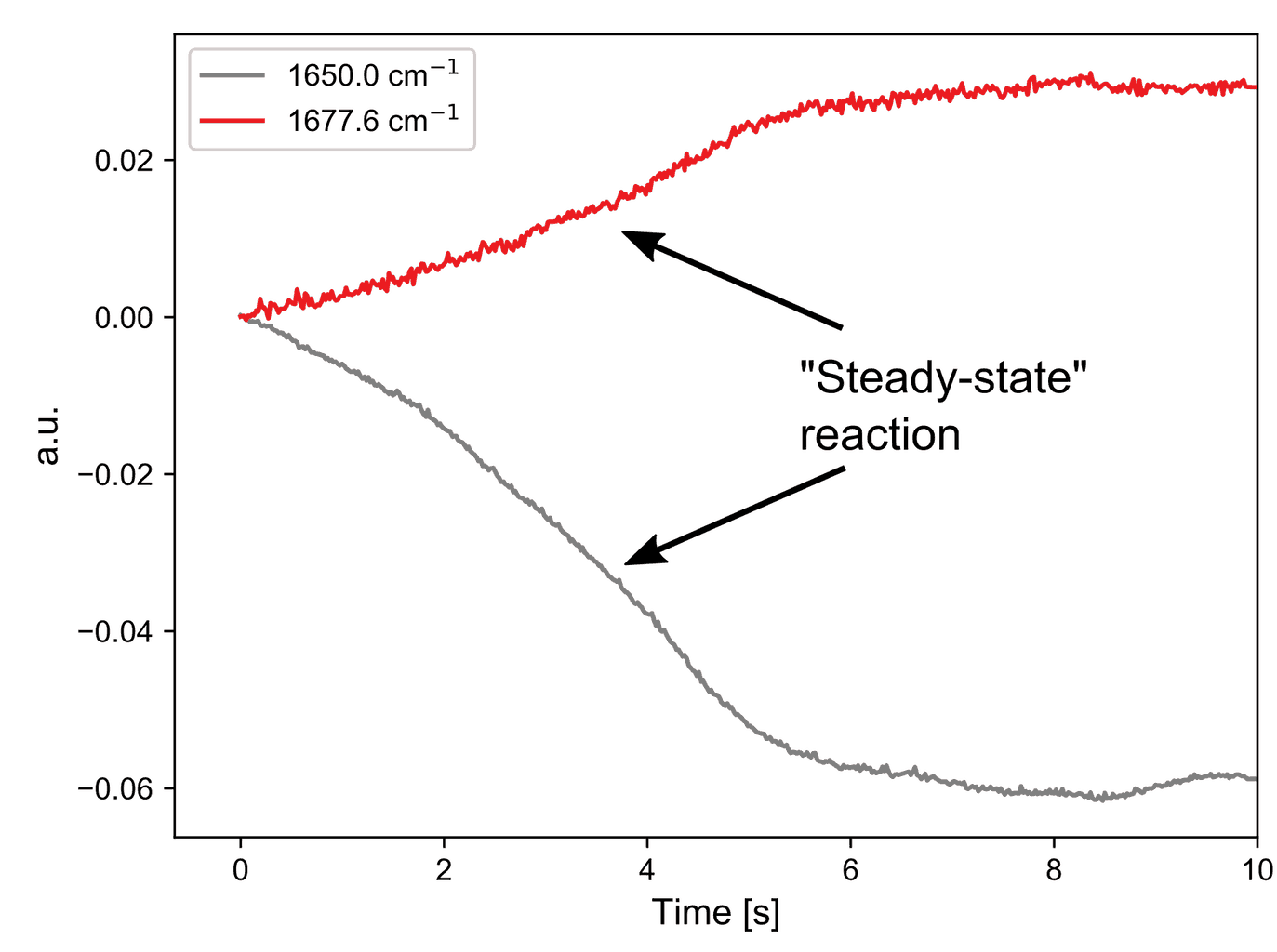
Both A and C have double bonds whose vibrational frequencies are affected by the substituents and this can be followed on a millisecond timescale. The kinetic traces at two points in the spectra clearly show that the simple hand pumped syringe setup is the limiting factor in resolving this reaction.
The first 1.5 seconds are dominated by movement of the solution during injection. However, when the solution is stopped, a bi-exponential decay can be seen, which is indicative of a first order reaction mechanism.
A thorough investigation would rely on mechanical injection and stopping to minimize changes that are not due to the reaction.
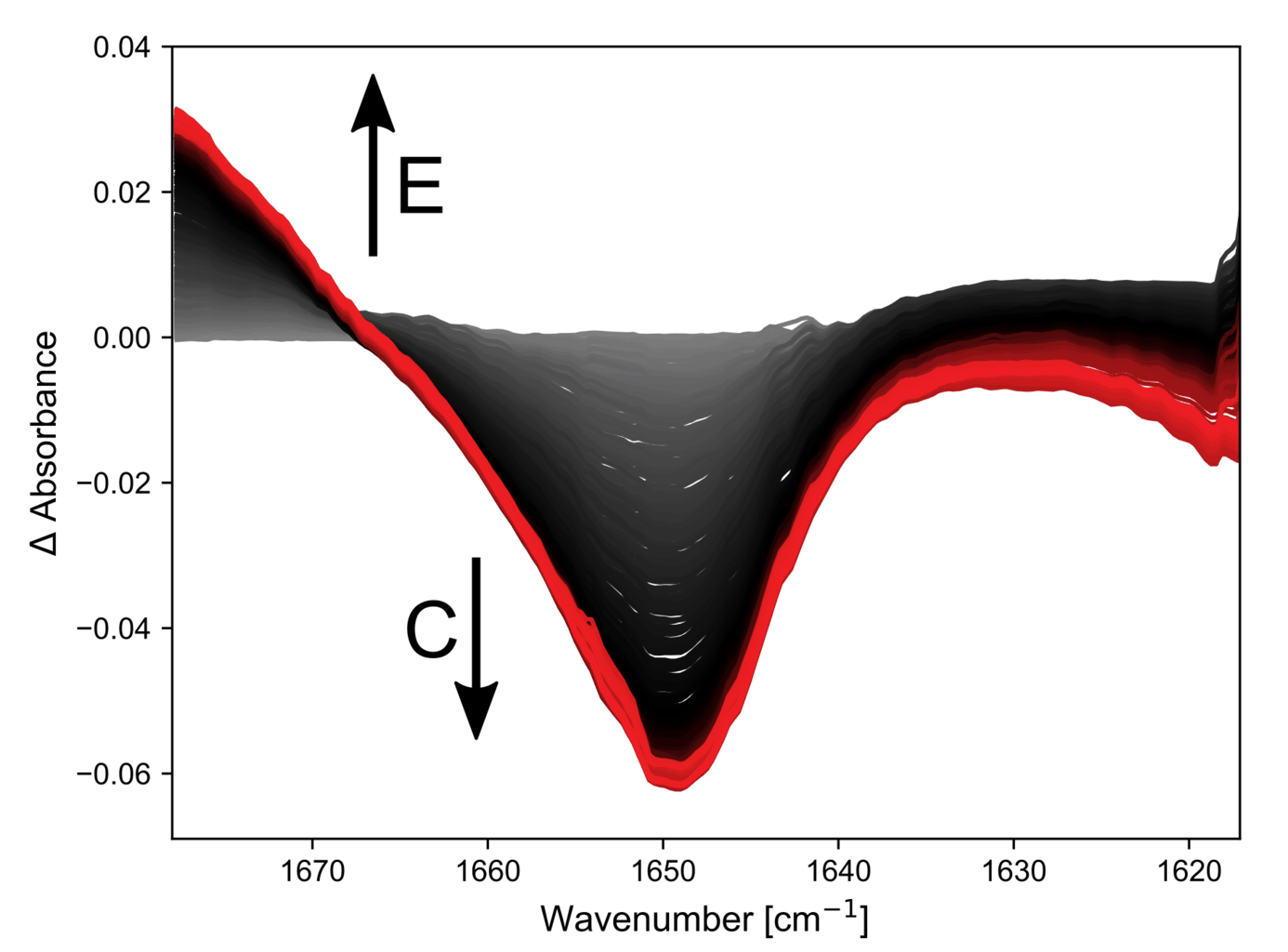
Next, the second reaction step was investigated. Here the band of intermediate C formed earlier disappears and a new double bond with a higher vibrational frequency is formed (product E).
The kinetic traces again show some syringe movement followed by an exponential decay. This reaction appears to take longer to complete but a rigorous kinetic study is needed to determine this with accuracy.
A note on time resolution
The time resolution of the IRis-F1 depends on the measurement mode. Using the faster, triggered, “time resolved” mode, we have shown measurements of full spectra to be possible in less than a microsecond. Here we use the more traditional “static” mode, where we first acquire a background spectrum, followed by a series of sample spectra, until the experiment is manually stopped.
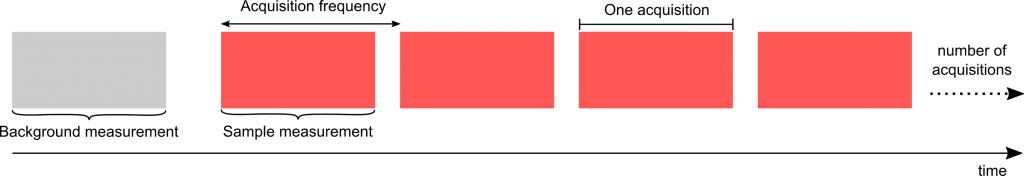
The limiting factor in acquisition frequency is currently how fast data can be saved and processed. This leaves a huge scope for increase in acquisition frequency, and therefore time resolution, by improving the computing power and software. In a previous experiment we measured at 40 spectra per second.
Improvements in software have already made it possible to acquire at 150 spectra per second and this number is increasing month by month. Since the integration time stays the same, this also improves the signal to noise ratio achieved.
We believe that with the IRis-F1 the limiting factor for time resolution in for stopped flow experiments is the flow equipment and not the spectrometer.

