Contents
Raphael Horvath1), Pit Boden2), Sophie Steiger2), Andreas Hugi1) Markus Gerhards2)
1) IRsweep AG, Laubisrütistr. 44, 8712 Stäfa, Switzerland
2) TU Kaiserslautern, Gottlieb-Daimler-Straße 47, 67663 Kaiserslautern, Germany
In 2020, we had the opportunity to visit the research labs of Prof. Markus Gerhards at the Technical University of Kaiserslautern and to work with him and his team. We were in the process of setting up a collaboration when Prof. Gerhards tragically and unexpectedly passed away. Sadly, we never finished some of the most exciting parts of our project, however, during our initial visit we acquired data on a pair of transition metal complexes which we would like to present here.
Background
The study of the interactions of materials with light is an important field, underpinning technologies such as water splitting, photovoltaics, display technologies, green catalysis, and more. When a molecule absorbs light, it effectively becomes a different compound which can undergo a chemical reaction (photochemistry) or return to its original state by transferring the absorbed energy (photophysics).
The metal complexes examined were [Cr(tpe)2](BF4)3 (tpe = 1,1,1-tris(pyrid2-yl)ethane) and [(2-(Diphenylphosphino)pyridine)(tris(4-fluorophenyl)phosphine)2Cu2I2]). The properties of these compounds have been published in detail, since they are promising candidates as, for example, photocatalysts and active materials in organic light emitting diodes.1,2 Here, they will be referred to as Cr complex and Cu complex, denoting the metal centers they are based on.
This application note describes the experiment carried out with the team at Kaiserslautern and the results obtained. Since the lifetimes of the studied compounds were known to be in the low microseconds, this work is a good way to push the limits of the IRis-F1 spectrometer in terms of time-resolution.
Setup
The complete setup from unpacking to first results took around 3-4 hours. The results shown here were obtained on the second day of measurements. This was in a large part possible thanks to the dedication of the team at Kaiserslautern.
The complexes investigated are shown in Figure 1. They were pressed in KBr disks and mounted in the sample compartment of the IRis-F1. The IR beam passing through the sample was 3 mm in diameter while the UV beam was approximately 5 mm in diameter to ensure uniform excitation. Each measurement consisted of 2000 shots and took 7 minutes to complete.
Figure 1. (left) Structures of [Cr(tpe)2](BF)3 and (right) [(2-(Diphenylphosphino)pyridine)(tris(4-fluorophenyl)phosphine)2Cu2I2]).
Excitation was carried out with a HY 750 Nd:YAG laser operating at 355 nm (Lumonics). A beamline of UV fused silica mirrors was used to bring the laser onto the sample through an antireflection-coated silica window mounted on the sample compartment door. Stray light was prevented from reaching the detector by use of an antireflection-coated germanium window.
Acquisitions were taken for 0.5 ms, of which 0.25 ms was designated as the background. The spectral resolution is set to 4.8 cm-1. Normally, the time resolution of IRis-F1 is limited to 1 µs as processing the data to shorter timescales can cause line broadening. Here, we processed to a time resolution of 250 ns. The broadband absorbers investigated for this experiment present a good target to explore sub-microsecond time resolutions.
Results
The Cr complex was measured at a center wavelength of ca. 1620 cm-1 and the results are shown in the Figure 2.
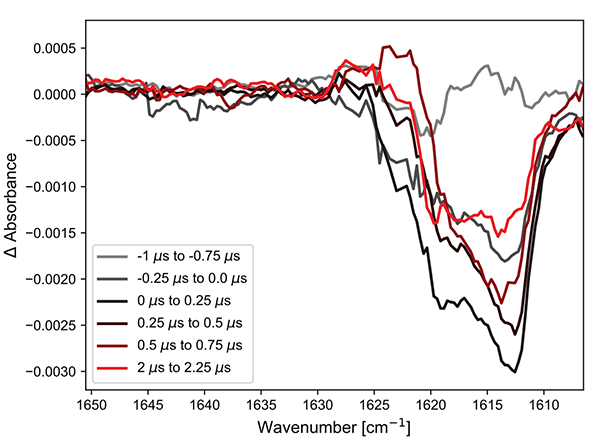
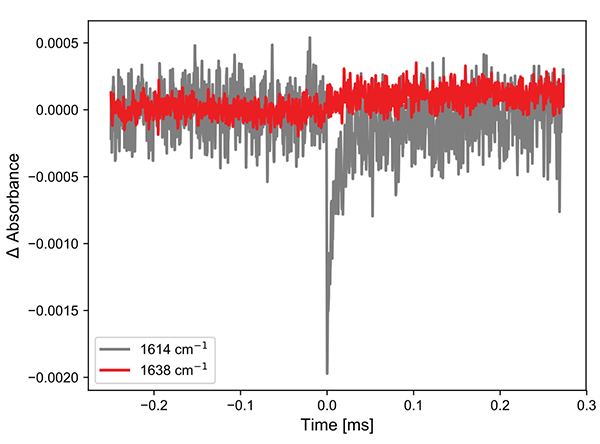
Figure 2. (left) Excited state infrared spectra of the Cr complex after 355 nm excitation. (right) Kinetics at two points in the spectra.
The left panel shows the spectra with an integration time of 250 ns per spectrum. Even at such short times, the bleach of the ground state is clearly visible. The shift of this band is to lower wavenumbers and therefore lies outside the window of observation. However, a small rise can be observed at ca. 1640 cm-1, which shows up well in the kinetic trace. The return to the ground-state follows a biexponential decay. These results are consistent with those published previously.
The Cu complex (Figure 3) shows both positive features and ground state bleach, as expected for the spectral region at 1240 cm-1. The kinetics can be fitted to a lifetime of 3 μs. This is a rather long lifetime for a compound of this type and matches with the previously reported result.
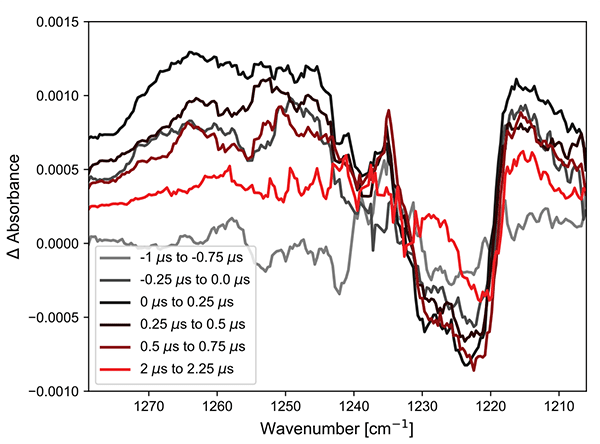
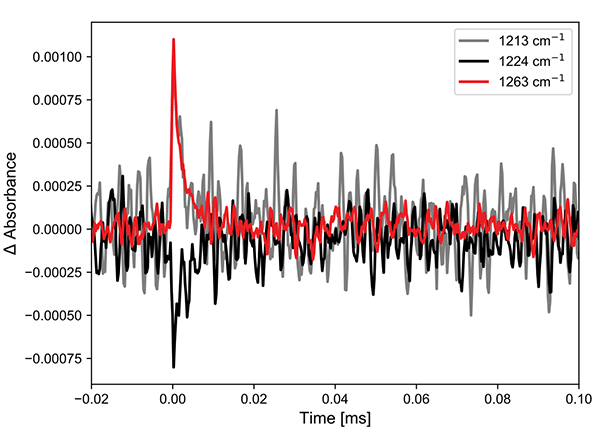
Figure 3. (left) Excited state infrared spectra of the Cu complex after 355 nm excitation. (right) Kinetics at two points in the spectra.
So far, we have only considered a single line for the analysis. However, since the IRis-F1 gives many lines, and the standard deviation of each line is also known, it makes sense to consider whole spectra in the analysis. The following analysis shows the result of a statistical evaluation, where the time-evolution of the entire spectrum is considered to get the kinetic trace. The result is far better signal to noise ratio compared to when only a single line is used (see Figure 4).
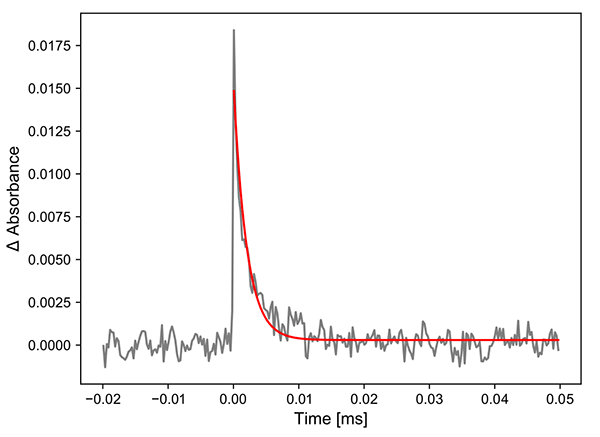 Figure 4. Kinetics of the statistically averaged kinetic traces of the Cu complex.
Figure 4. Kinetics of the statistically averaged kinetic traces of the Cu complex.
Summary
We successfully measured the spectra of the excited states of the two metal complexes shown above. Thanks to the efforts of the Gerhards group, the whole measurement campaign took less than three days, from initial setup, to collecting the results, to packing up. Excellent time-resolutions of 250 ns were achieved, and the actual data collection could be finished in 7 minutes. Finally, we would like to once again express our gratitude to Prof. Gerhards. In the short time we knew him, he often took time out of his busy schedule to meet with us, during our visit and later in follow-ups. He was always extremely engaged, questioning methods and data with the aim of improving them, and encouraged his co-workers to do the same. He was an inspiring collaborator, and we are fortunate to have shared a project with him, even if it was sadly cut short.
References
- S. Treiling, C. Wang, C. Förster, F. Reichenauer, J. Kalmbach, P. Boden, J. P. Harris, L. M. Carrella, E. Rentschler, U. Resch-Genger, C. Reber, M. Seitz, M. Gerhards, K. Heinze, Angew. Chem. Int. Ed. 2019, 58, 18075.
- J. M. Busch, D. M. Zink, P. Di Martino-Fumo, F. R. Rehak, P. Boden, S. Steiger, O. Fuhr, M. Nieger, W. Klopper, M. Gerhards, S. Bräse, Dalton Trans. 2019, 48, 15687.



