Following kinetics with mid infrared (mid-IR) spectroscopy allows protein chemists to better understand how proteins fold and to obtain information about secondary structure changes involved in the folding process. Stopped-flow is a popular technique for these studies where reagents are rapidly mixed and the consequent reactions can be studied with few milliseconds dead time (more information here and here). Traditionally, the stopped-flow instrument is coupled to a Fourier Transform InfraRed (FT-IR) spectrometer. Despite the improvement in FT-IR technology and use of step scan acquisition, the main limitation of such set-ups for rapid kinetics is often the time resolution of the FT-IR spectrometer. The fastest acquisition speed is often in the 30-50 ms range (or even longer), which is much longer than the few milliseconds dead time (with FT-IR cell) of the BioLogic SFM stopped-flow instrument used in this work. Hence, rapid kinetics shorter than 100-200 ms cannot be observed with such technology.
At a glance: Figures of merit
- IR-spectra with 4µs acquisition time
- 1000 spectra/second continuous reaction monitoring at 0.3 cm-1 resolution
- 10-3 AU noise floor in single shot
- 5 ms dead time
- 75-100 µl of sample per shot
- Umbilical volume = 200 µl
- Single, double and triple mixing
- 100 µm to 500 µm light path
A second limitation is the signal to noise ratio of the spectrometer as it is directly linked to the acquisition speed. Commercial FT-IR spectrometers with faster acquisition speeds often require averaging over many stopped-flow shots to improve signal to noise ratio which can prove problematic when working with the precious or concentrated samples required in the IR region.
The IRis-F1 spectrometer from IRsweep is a dual comb spectrometer specifically designed for rapid measurements that allows recording mid-IR spectra at 4 µs time resolution in a single stopped-flow shot. The aim of this application note is to show how the BioLogic SFM can be connected to the IRis-F1 from IRsweep, to follow reactions in the millisecond time scale, and to observe never-seen-before mid-IR reactions.
What is dual comb spectroscopy?
The principles of dual-comb spectroscopy are fundamentally different from conventional Fourier transform – or dispersive spectrometers. Broadband infrared beams (frequency combs) emitted by two closely matched laser sources are superimposed on a single detector element after passing through the sample. The wavelengths of the detected light are resolved based on the frequency of the radio-frequency signal observed at the detector. Since no moving parts are needed, a full infrared spectrum can be recorded in only four microseconds in a single experiment. Millisecond reaction dynamics as studied by the stopped-flow technique can hence be easily followed based on infrared spectral signatures at superior signal to noise ratios, as is demonstrated in this application note. Being based on high power laser sources, the dual-comb spectrometer also features high brightness, allowing for measurements in strongly absorbing solvents with high spectral and time resolution.
 BioLogic SFM 4000 and IRsweep IRis-F1
BioLogic SFM 4000 and IRsweep IRis-F1
Experimental setup
An SFM-4000 was used in this application note together with an IRis-F1 spectrometer (see Figure 1). The SFM-4000 has 3 mixers and 4 syringes driven by independent stepping motors to give the user full control of volume, injection speed and mixing ratio. The SFM’s FT-IR accessory is fully compatible with the Iris-F1 sample compartment.
The flow cell uses CaF2 windows and the user can freely select light paths from 100 μm to 500 μm by changing a spacer between windows. For the purposes of this application note a 100 µm spacer was installed which corresponds to a dead volume (from last mixer to the center of the cell), of 15µl, so to a dead time of 5 ms using a 3 ml/s flow rate. The last mixer is directly integrated into the FT-IR accessory to minimize the dead volume.
The SFM-4000 main body is connected to the FT-IR flow cell using an umbilical connector. This umbilical brings the solutions coming from the exit of mixer 2 and from syringe 4 to the last mixer for the final mixing stage. The instantaneous stop of the flow is ensured by the synchronization of the hard-stop valve with the motors stop. The synchronization between the stopped-flow push and the IRis-F1 is made using a 5V TTL trigger.
Secondary structure change in β-lactoglobulin
The fast transition from β-sheet to α-helix in β-lactoglobulin secondary structure has been described by Gerwert et al.(DOI) using trifluoroethanol as denaturant agent. This fast reaction is used here to demonstrate the performance of the SFM-4000 and IRis-F1 coupling.
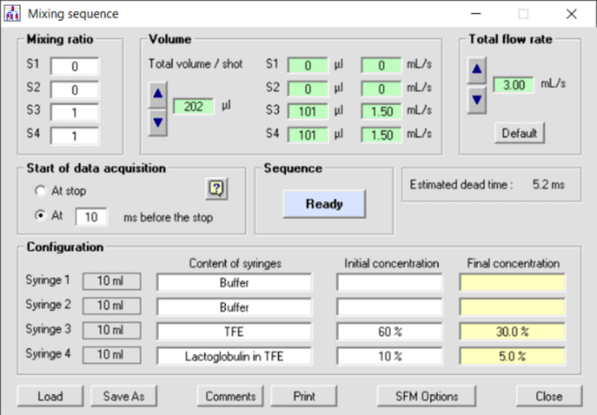 Figure 2: Mixing sequence in BioKine – software used for the β-lactoglobulin reaction
Figure 2: Mixing sequence in BioKine – software used for the β-lactoglobulin reaction
The transition in secondary structure is initiated by mixing a solution of 25 mg/ml β-lactoglobulin in 10 %V trifluoroethanol/20 mM DCl in D2O with 60 %V trifluoroethanol/20 mM DCl in D2O at a ratio of 1:1 (see Figure 2), resulting in a concentration step of trifluoroethanol from 10 %V to 35 %V.
Deuterated water and hydrochloric acid were used to avoid the strong background absorbance of H2O at 1650 cm-1. For the experiments, 100 µl of each solution were injected at 3 ml/s resulting in a 5 ms dead time.
Spectra were continuously acquired every 4 µs for 130 ms. Pre-trigger spectra, corresponding to the initial state of the reaction, were used as the background for absorbance spectra. In post-processing, spectra were co-averaged over 1 ms and spectral averaging over 3 cm-1 was performed.
Results
Figure 3 shows the evolution of the IR spectra measured during the first 10 ms. Four stopped-flow experiments were co-averaged and baseline drifts were corrected by subtracting the absorbance at 1623 cm-1. The positive band at 1660 cm-1 can be attributed to the formation of a-helix structures, while the negative band at 1634 cm-1 corresponds to the consumed b-sheet. At short time time-scales below 3 ms, a small positive band at ~1637 cm-1 can be observed that has been attributed to an intermediate species by Gerwert and co-workers. For later times above 7 ms, this small positive band is superimposed by the negative b-sheet band.
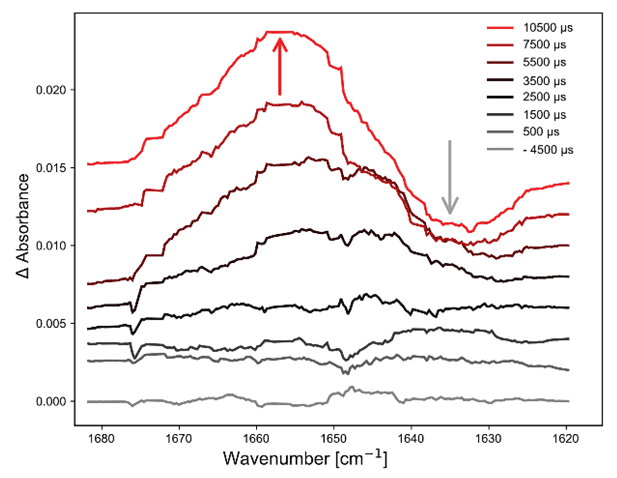
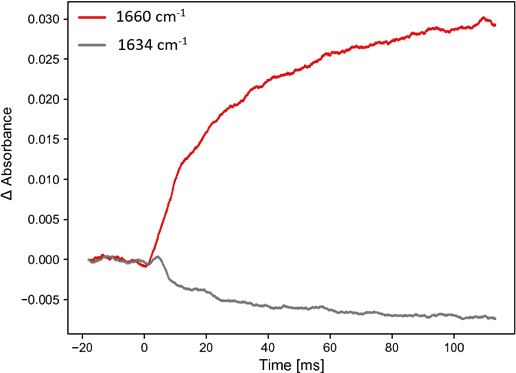
Figure 3. Spectral signatures of secondary structure change in b-lactoglobulin. Left: spectra recorded during first 10 ms, consecutive offset for visualization purposes. Right: evolution of absorbance at 1660 cm-1 and 1635 cm-1.
Conclusions
The BioLogic SFM can be coupled easily to the IRis-F1 dual comb spectrometer using the standard FT-IR and an umbilical connector accessory. Sub-10 ms bio-chemical reaction kinetics can be studied in the mid-IR region where traditional FT-IR experiments are impossible with commercial instruments.
With the IRis-F1, absorbance spectra from a single stopped-flow shot are acquired at a noise floor of 10-3 AU (absorbance units) with 1 ms time resolution. Sensitivity can be further increased by co-averaging multiple shots or reducing the time resolution.
Only two SFM-4000 syringes were used for the described experiments, but the two additional syringes can be useful if users wish to program automatic concentration studies or double mixing experiments. In such cases, one umbilical flow line is used as a delay line to age the first mixing. SFM-2000 and SFM-3000 can also be coupled to the IRis-F1 spectrometer.This application note was written in collaboration with BioLogic, France, www.biologic.net




