Understanding the mechanism of a chemical reaction is an important step away from trial-and-error-based reaction optimization. By studying the structures of intermediates, for how long they exist, and the ways in which they can and cannot react, reactions can be intelligently designed and controlled.
This application note explores the use of the IRis-F1 spectrometer in infrared stopped flow experiments. First we investigate the hydrolysis of methyl chloroacetate (MCA) and this will be followed by the refolding of ubiquitin, which is expected to be a more challenging measurement.
Stopped-flow
Stopped-flow is a powerful technique to study reactions on a millisecond to minute timescale.
Simply speaking, it works by rapidly mixing reagents before a spectroscopic cell, as depicted in the figure.
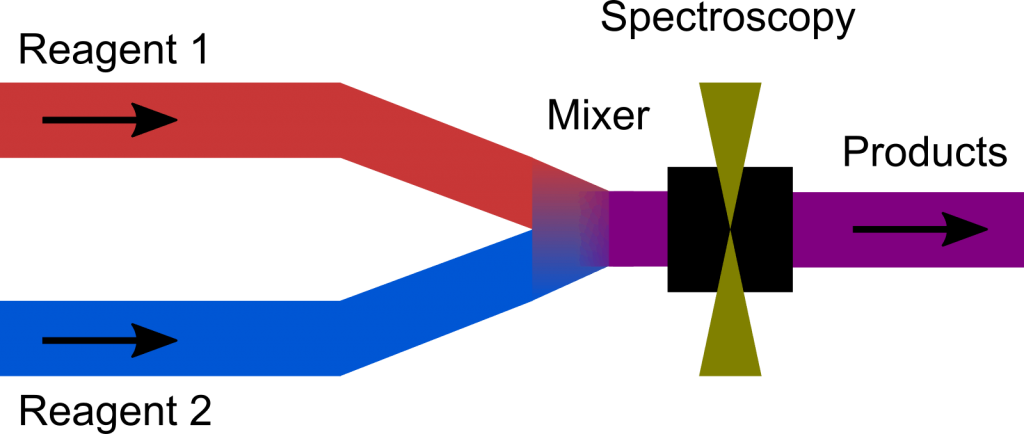
For this set of experiments, we partnered with TgK Scientific, a leader in stopped-flow IR equipment. The syringe drive unit of the SF-73 stopped-flow system is shown in the picture (above). It connects via an umbilical to a spectroscopic cell with CaF2 windows and a path-length of 100 µm.
For the MCA experiment, a background was acquired of the pure solvent and for the ubiquitin experiment a background was acquired of the already-reacted mixture. This was necessary to subtract the signal from the changing solvent environment.
The IRis-F1 was triggered from the SF-73 through a TTL signal sent through a BNC connection and user control happened through KinetaDrive. This way, shots are synchronized to data collection providing a consistent reference to time zero and the convenience and reassurance of not missing data. Typically, spectra are integrated for 0.5 ms and acquired every 4.5 ms.
Hydrolysis of methylchloroacetate
The scheme for the hydrolysis of MCA is shown below.
This reaction is often carried out as a test for stopped flow systems and typically hydroxide concentrations of 0.1 M or less are used. Here, we used a concentration of KOH of 0.5 M to speed up the reaction. The measurement was carried out in D2O.
The results of a single shot of the hydrolysis reaction are shown in the figures. The spectra (below) show the disappearance of a weak MCA band around 1650 cm-1 and the appearance of a new band belonging to the formed acetate salt at 1602 cm-1. Excellent signal to noise (S/N) ratios are seen throughout the spectra and despite the low intensity of the MCA band, it can be fitted well with a mono-exponential decay. A lifetime of 53 ms is found for this process at room temperature.
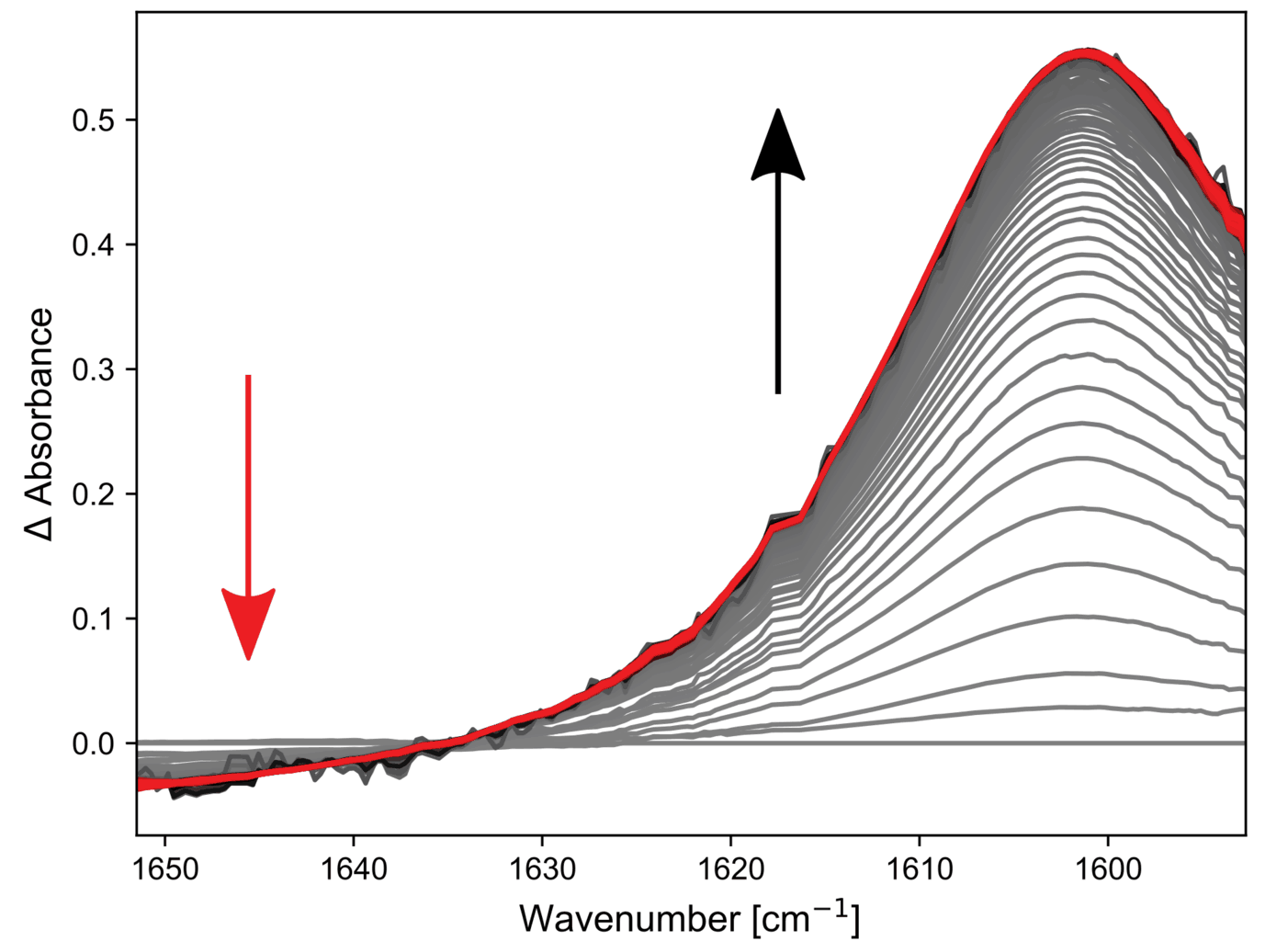 Spectra of the hydrolysis reaction of MCA. 4.5 ms per spectrum.
Spectra of the hydrolysis reaction of MCA. 4.5 ms per spectrum.
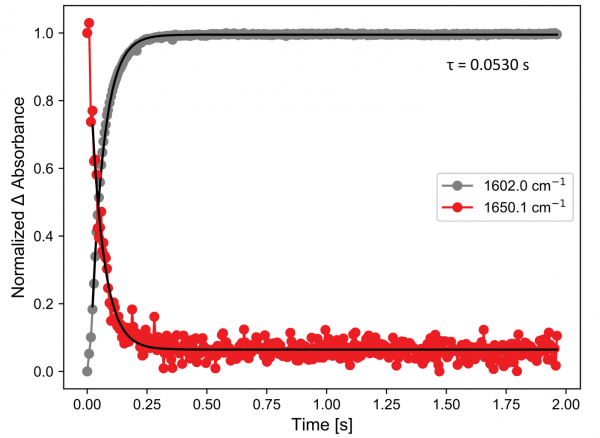 MCA hydrolysis: kinetics at two spectral points
MCA hydrolysis: kinetics at two spectral points
Folding of ubiquitin
The example of MCA shows a strong absorbance response. A more challenging experiment, inspired by the work of Lendl and coworkers, involves the exposure of the protein ubiquitin to a change in solvent (20:80 methanol:D2O → 55:45 methanol:D2O). The resulting β-sheet to β-turn folding can be observed in the Amide I region of the IR spectrum. We used CH3OD for this experiment, to avoid the strong background absorption associated with the OH stretch of methanol in this spectral region.
The difference spectrum shows a depletion at ca. 1635 cm-1 and a new band, with two features around 1660 and 1650 cm-1 and a lifetime of 1.46 s. These band positions are consistent with values in the literature, where a shift was reported rather than a difference spectrum. Here the time resolution is approximately 20 ms and even for the small spectral changes observed, the S/N ratios remain good.
The reproducibility of the reagent delivery by the SF-73 and the synchronization of time by triggering, allow the averaging of multiple shots to further improve the S/N ratio. Alternatively, the improved S/N provides the opportunity to rapidly repeat a reaction under different conditions, such as temperature, enabling wider reaction parameter space to be mapped in shorter times and with low sample consumption.
Conclusion

Stopped-flow is a powerful tool to investigate the IR spectra of evolving and transient species and the monitor reactions on the timescale of milliseconds to minutes. It allows for precise, efficient and rapid mixing of a wide range of samples at low volumes, controlled temperatures and, if necessary, at anaerobic conditions.
We have successfully investigated chemical and biochemical reactions with this technique and have shown that good S/N ratios can be obtained even for weak signals and with single repetitions. Combining dual comb spectroscopy with stopped-flow opens up investigations of new reactions and processes that were previously difficult or impossible to measure in the IR.



