Spectroscopy on proteins

Infrared spectroscopy is well suited to study proteins in solution. It is regularly used, for example, in secondary structure analysis and protein dynamics studies. FTIRs are currently widely used for these applications, but impose significant limitations in terms of speed, brightness and achievable signal-to-noise ratio per time.
The IRis-F1 dual-comb spectrometer overcomes such limitations by using broadband laser sources and simultaneously acquiring the whole laser spectrum within less than a microsecond.
Laser modules covering different spectral bands of interest for protein studies – such as the Amide bands – are available. Users can exchange laser modules without tools to switch from one spectral band to another.
Protein dynamics
Step-scan FTIR spectroscopy is the standard method to analyze protein reactions, but it requires the reaction to be massively repeatable, strongly limiting the amount of systems that can be studied. It is also very time consuming, as measurements take hours to several days.
The IRis-F1 simultaneously acquires spectral and temporal resolution without the time limitation of a moving mirror, and therefore enables single-shot studies on non-repeatable samples, such as vertebrate rhodopsins and GPCRs.
Due to the high brightness of the laser source, stronger signals than in FTIRs can be achieved by increasing the sample interaction length, which at the same time often simplifies sample preparation.
The multi-heterodyne detection scheme of the lasers enables sub-microsecond time resolution, and makes it possible to resolve sub-mOD sized features in a single acquisition. If reactions are repeatable, features below 10 µOD can be resolved in acquisition times of less than a minute.
As the full heterodyne spectrum is recorded in each experiment repetition, it becomes possible to improve signal-to-noise ratios with few averages, and observe sample degradation at the same time. Step-scan techniques, on the other hand, typically require thousands of repetitions without providing any insight into sample degradation during acquisition.
Secondary structure analysis
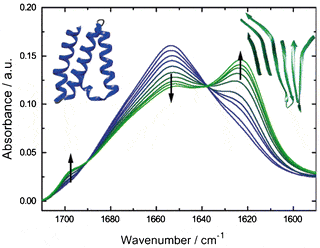
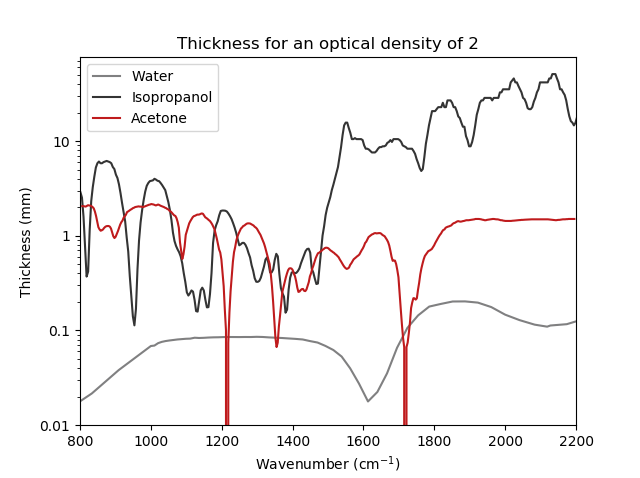
The secondary structure of proteins can be well studied in the infrared, as alpha helix, beta sheet and random coil have characteristic signatures in the Amide. The amount of each secondary structure can be quantified and transitions can be resolved. This is true of all infrared spectrometers, but the low brightness of the thermal light source of FTIRs is a severe limitation when studying proteins in solvents. This is especially the case in water, which absorbs very strongly in the Amide band.
With the IRis-F1, the sample thickness can be increased about 5x from about 8 µm to about 40 µm, boosting the total signal and simplifying sample preparation. When different solvents – such as heavy water or other spectral regions – are used, much thicker path lengths can be realized. Measurement time with the IRis-F1 is also reduced by about a factor of 100 compared to FTIRs for similar signal-to-noise ratios.
Stark spectroscopy
When studying the response of molecules to electric fields in the IR, long acquisition times and dielectric breakdown are regular problems. Also, high concentrations of analytes often have to be used to keep measurement times reasonable.
With the IRis-F1, measurement times can be reduced by about a factor of 100, allowing lower analyte concentrations and even parameter sweeps. Moreover, thicker samples can be used, boosting the signal even more.



